Deferoxamine Sodium Sterile
Deferoxamine Sodium Sterile
Deferoxamine Sodium Sterile Manufacturers in India, RAL ( Rajasthan Antibiotics Ltd.) produces, distributes, and exports a wide range of premium pharmaceutical APIs all over the world. RAL-Life ( Rajasthan Antibiotics Ltd.) is the leading Deferoxamine Sodium Sterile Manufacturer in India. All of our APIs and intermediates are produced in FDA-approved, top-notch, cutting-edge manufacturing facilities that follow cGMP standards.
RAL-Life ( Rajasthan Antibiotics Ltd.) is largest Deferoxamine Sodium Sterile Manufacturers in India. RAL-Life ( Rajasthan Antibiotics Ltd.) is a fast growing integrated pharmaceutical company which commenced its commercial production in 1991 and has increased in both size and stature to become one of the country’s largest producers of the Sterile API’s (Active Pharmaceutical Ingredients). Besides being the first company in India to produce Ampicillin Sodium Sterile, RAL-Life ( Rajasthan Antibiotics Ltd.) also pioneered the manufacturing of Sterile Anti-Ulcer drugs namely Omeprazole Sodium Sterile and Pantaprazole Sodium Sterile for which it has won worldwide acceptance. RAL ( Rajasthan Antibiotics Ltd.) established the Manufacturing of Deferoxamine Sodium Sterile in India, for which it has won universal recognition. The Company has a well-established clientele in India as well as all over the globe.
Deferoxamine Sodium Sterile, also known as deferoxamine mesylate, is a medication used to treat iron overload in the body. Iron overload can occur due to various reasons, including hereditary disorders such as beta-thalassemia major, sickle cell disease, and hereditary hemochromatosis, as well as chronic transfusion therapy. When there is an excessive amount of iron in the body, it can lead to serious health problems such as organ damage and death. Deferoxamine Sodium Sterile works by binding to excess iron in the bloodstream, which is then excreted through urine and feces.
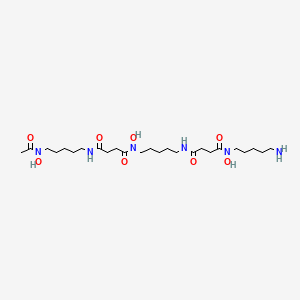
| C25H48N6O8 | |
|---|---|
 |
|
| CAS No. | 70-51-9 |
| HSN Code | 30049099 |
| Standard packs include 1 kg, 2 kg, 4 kg, 5 kg, and 10 kg (or) dependent on the needs of the customer. | |
Deferoxamine Sodium Sterile Manufacturers in India
Regarding sterile sodium Deferoxamine:
Deferoxamine Sodium Sterile is a synthetic chelating agent that is administered through injection as a slow infusion into a vein or under the skin. It is available in the form of a powder that is mixed with sterile water before administration. The dosage and duration of treatment with Deferoxamine Sodium Sterile will depend on the patient’s condition, age, weight, and overall health.
Deferoxamine Sodium Sterile is primarily used to treat iron overload in patients with beta-thalassemia major, sickle cell disease, and hereditary hemochromatosis. Beta-thalassemia major is a genetic disorder that affects the production of hemoglobin, a protein in red blood cells that carries oxygen throughout the body. Sickle cell disease is another genetic disorder that affects the structure of hemoglobin, causing it to form into a sickle shape and block blood flow to various organs. The condition known as hereditary hemochromatosis makes the body absorb an excessive amount of iron from the diet, resulting in iron overload.
Deferoxamine Sodium Sterile may also be used to manage acute iron poisoning in some cases. Iron poisoning can occur when a person ingests large amounts of iron-containing supplements, medications, or other substances. It can cause severe symptoms such as vomiting, abdominal pain, and diarrhea, and can even be life-threatening. Deferoxamine Sodium Sterile is sometimes used as an antidote to help remove excess iron from the body and prevent further damage.
When taken as prescribed, Deferoxamine Sodium Sterile is generally safe and well-tolerated. However, it can have negative effects, just like all drugs. Common side effects of Deferoxamine Sodium Sterile include nausea, vomiting, abdominal pain, diarrhea, and rash. These side effects are usually mild and go away on their own within a few days. Patients who experience any severe or persistent side effects while taking Deferoxamine Sodium Sterile should contact their healthcare provider immediately.
RAL is a well-known, internationally recognised maker of Specialty Chemicals and Pharmaceuticals in India. Pharmaceutical APIs, formulations, and other products are made from chemicals that we produce.
In rare cases, serious side effects such as allergic reactions, low blood pressure, and kidney damage can occur. Patients who experience any of these symptoms while taking Deferoxamine Sodium Sterile should seek immediate medical attention.
Deferoxamine Sodium Sterile is available by prescription only and should only be used under the guidance of a healthcare professional. Pregnant or breastfeeding women should consult with their healthcare provider before taking Deferoxamine Sodium Sterile. The medication may interact with other medications, so patients should inform their healthcare provider of all medications they are currently taking before starting treatment.
Deferoxamine Sodium Sterile can be taken with or without food, but patients should avoid consuming foods that are high in iron while taking the medication. Foods that are high in iron include red meat, liver, beans, and spinach.
Deferoxamine Sodium Sterile should be stored in a cool, dry place away from direct sunlight and heat. The medication should not be frozen. Once mixed with sterile water, Deferoxamine Sodium Sterile should be used immediately or stored in a refrigerator for up to
| Side effects from deferoxamine injection | |
|---|---|
| Deferoxamine Sodium Sterile is a medication used to treat iron overload in conditions such as thalassemia, sickle cell disease, and hemochromatosis. Some of the most common side effects of Deferoxamine Sodium Sterile include: | Injection site reactions, such as redness, swelling, or pain at the injection site |
| Skin rash or itching | |
| Nausea, vomiting, or diarrhea | |
| Fever or chills | |
| Muscle or joint pain | |
| Deferoxamine Sodium Sterile can cause serious side effects such as allergic reactions, low blood pressure, or damage to the retina (the part of the eye that detects light). |
Deferoxamine sodium sterile FAQ
What is deferoxamine sodium sterile used for?
Deferoxamine sodium sterile is used to treat iron overload in patients who receive regular blood transfusions.
How is deferoxamine sodium sterile administered?
Deferoxamine sodium sterile is usually administered through a subcutaneous infusion pump, which delivers the medication through a small needle placed under the skin.
What are the potential side effects of deferoxamine sodium sterile?
Common side effects of deferoxamine sodium sterile include nausea, vomiting, diarrhea, stomach pain, and skin irritation at the infusion site. Rare but serious side effects can include kidney damage, hearing loss, and vision loss.
How often do patients receiving deferoxamine sodium sterile need to undergo monitoring?
Patients receiving deferoxamine sodium sterile should undergo regular monitoring to ensure the medication is working effectively and to monitor for potential side effects or complications. The frequency of monitoring will depend on the patient’s individual needs and the severity of their iron overload.
Can patients become pregnant while receiving deferoxamine sodium sterile?
Patients should inform their healthcare provider if they become pregnant or plan to become pregnant while receiving deferoxamine sodium sterile, as the medication may have potential risks for the developing fetus.
What should patients do if they miss a dose of deferoxamine sodium sterile?
If a patient misses a dose of deferoxamine sodium sterile, they should contact their healthcare provider immediately for guidance on how to proceed.
Are there any potential drug interactions with deferoxamine sodium sterile?
Patients should inform their healthcare provider of all medications and supplements they are taking, as some medications can interact with deferoxamine sodium sterile and affect its effectiveness or increase the risk of side effects.
How long do patients typically need to receive treatment with deferoxamine sodium sterile?
The duration of treatment with deferoxamine sodium sterile will depend on the patient’s individual needs and the severity of their iron overload. Some patients may need to receive treatment for several years or even for the rest of their lives.
Can deferoxamine sodium sterile be used to treat iron overload in patients who do not receive blood transfusions?
Deferoxamine sodium sterile is specifically indicated for the treatment of iron overload in patients who receive regular blood transfusions. It is not typically used to treat iron overload in patients who do not receive blood transfusions.
How is the dosage of deferoxamine sodium sterile determined?
The dosage of deferoxamine sodium sterile is determined based on the patient’s weight, the severity of their iron overload, and other individual factors. The dosage may need to be adjusted over time based on the patient’s response to the medication.
How should deferoxamine sodium sterile be stored?
Deferoxamine sodium sterile should be stored in a cool, dry place, away from direct sunlight and heat sources. It should not be stored in the refrigerator or freezer.
Are there any special precautions that patients should take while receiving treatment with deferoxamine sodium sterile?
Patients should be careful not to expose the medication to moisture, as this can cause the medication to degrade. In addition, patients should be aware of the potential for medication errors or accidental overdose and should follow the prescribed dosage and administration instructions provided by their healthcare provider.
Registered Office
222A, 2nd Floor
Hemkunt Chambers, 89,
Nehru Place, New Delhi-110019
Corporate Office
M 134 , 2nd Floor, Opp.
Super Bazar, Connaught Place,
New Delhi – 110001
